How Network Meta-Analysis Informs Systemic Treatments for Atopic Dermatitis
Abstract
Network meta-analysis (NMA) is an increasingly popular statistical technique employed in clinical research. NMA is extremely appealing because it allows clinicians to compare a wide range of treatments used for the same condition in a single analysis. This is helpful in the clinic setting when deciding between treatment options for a patient with moderate-to-severe atopic dermatitis (AD). It allows us to help patients understand the relative efficacy of various medications.
In an ideal world, randomized clinical trials would be conducted comparing all possible relevant treatment options. In reality, that would not be practical or feasible, particularly in a rapidly expanding therapeutic field. While there are only a few systemic treatment options currently available for AD, that number is set to increase steadily over time. We only need to look to psoriasis and the myriad treatments approved over the last two decades to understand that planning head-to-head clinical studies for all comparisons is not realistic. NMA helps us circumvent this problem.
References
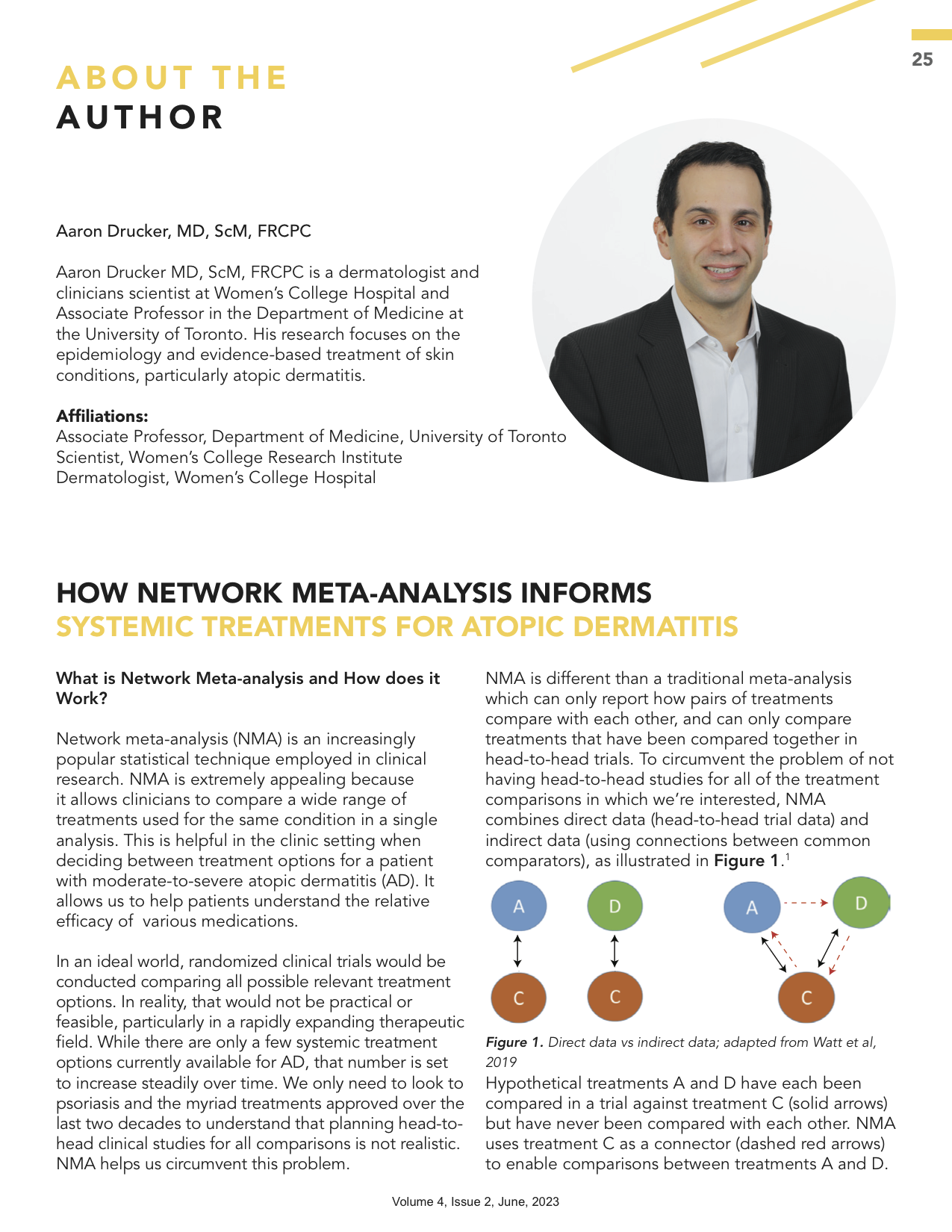
Watt J, Tricco AC, Straus S, Veroniki AA, Naglie G, Drucker AM. Research techniques made simple: network meta-analysis. Journal of Investigative Dermatology. 2019 Jan 1;139(1):4-12. doi:10.1016/j.jid.2018.10.028
Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, Kataoka Y, Chu CY, DiBonaventura M, Rojo R, Antinew J. Abrocitinib versus placebo or dupilumab for atopic dermatitis. New England Journal of Medicine. 2021 Mar 25;384(12):1101-12. doi:10.1056/NEJMoa2019380
Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, Bieber T, Thyssen JP, Yosipovitch G, Flohr C, Magnolo N. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet. 2020 Jul 25;396(10246):255-66. doi:10.1016/S0140-6736(20)30732-7.
Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, Prajapati VH, Lio P, Hu X, Wu T, Liu J. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatology. 2021 Sep 1;157(9):1047-55. doi:10.1001/jamadermatol.2021.3023
Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, Thaçi D, Chu CY, Hong HC, Katoh N, Paller AS. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. The Lancet. 2021 Jun 5;397(10290):2151-68. doi:10.1016/S0140-6736(21)00588-2.
Drucker AM, Ellis A, Jabbar-Lopez Z, et al. Systemic immunomodulatory treatments for atopic dermatitis: protocol for a systematic review with network meta-analysis. BMJ Open. Aug 29 2018;8(8):e023061. doi:10.1136/bmjopen-2018-023061
Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZ, Rochwerg B, Di Giorgio S, Arents BW, Burton T, Spuls PI, Küster D. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatology. 2020 Jun 1;156(6):659-67. doi:10.1001/jamadermatol.2020.0796.
Drucker AM, Morra DE, Prieto-Merino D, Ellis AG, Yiu ZZ, Rochwerg B, Di Giorgio S, Arents BW, Burton T, Spuls PI, Schmitt J. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatology. 2022 May 1. doi:10.1001/jamadermatol.2022.0455.
Williams HC, Schmitt J, Thomas KS, Spuls PI, Simpson EL, Apfelbacher CJ, Chalmers JR, Furue M, Katoh N, Gerbens LA, Leshem YA. The HOME Core outcome set for clinical trials of atopic dermatitis. Journal of Allergy and Clinical Immunology. 2022 Jun 1;149(6):1899-911. doi:10.1016/j.jaci.2022.03.017.
Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt JE. EASI,(objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012 Jan;67(1):99-106. doi:10.1111/j.1398-9995.2011.02719.x.
Lau KP, Agarwal P, Parente L, Marcello O, Lovas M, Van J, Vigod SN, Champagne T, Mohan T, Arents BW, Burton T. Development of a Website for a Living Network Meta-analysis of Atopic Dermatitis Treatments Using a User-Centered Design: Multimethod Study. JMIR Dermatology. 2022 Sep 26;5(3):e41201. doi:10.2196/41201.
Leshem YA, Bissonnette R, Paul C, Silverberg JI, Irvine AD, Paller AS, Cork MJ, Guttman-Yassky E. Optimization of placebo use in clinical trials with systemic treatments for atopic dermatitis: an International Eczema Council survey-based position statement. Journal of the European Academy of Dermatology and Venereology. 2019 May;33(5):807-15. doi:10.1111/jdv.15480.